Meanwhile the Australian Orthopaedic Association has been given a $13 million grant to run the National Joint Replacement Registry for the next four years.
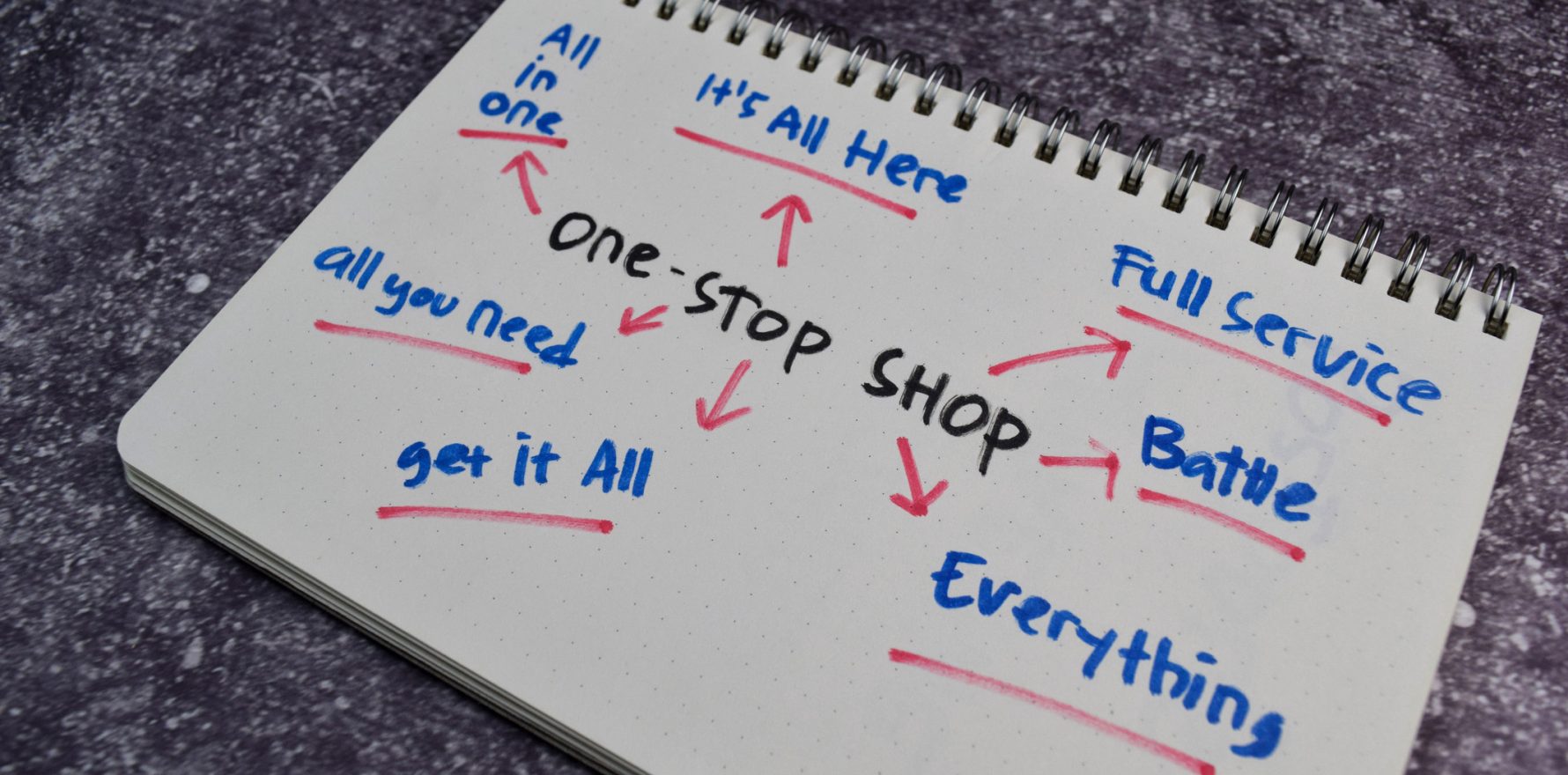
The Department of Health, Disability and Ageing is looking to streamline the fragmented regulatory and operating environment for clinical trials in Australia by building a one-stop shop for researchers via a single, national approvals and data system.
“The current regulatory and operating environment for clinical trials is fragmented and inefficient, with different rules, systems and processes in each state and territory,” said the DoHDA in a Request for Information released via AusTender recently.
“This puts a large administrative and regulatory burden on the medical research sector, especially for research that crosses state and territory borders.
“The National One Stop Shop (NOSS) program will streamline health and medical research through a single, national approvals and data system.
“This will make it easier for patients, researchers and sponsors to find, conduct and participate in clinical trials and research in Australia.”
The department is seeking information from suitably qualified vendors about cutting-edge technologies to support the NOSS ICT solution.
“This is a unique opportunity to help inform the Department’s approach to a transformative ICT solution that will improve health and medical research nation-wide and establish Australia as a global leader for clinical trials,” it said in the RFI documents.
About 95,000 Australians participate in a clinical trial annually and more than 1200 new clinical trials begin.
“Under NOSS, the ICT solution will enable capacity for at least 200,000 users (researchers, clinicians, sponsors, governments) and more than 8000 health and medical research applications per annum, each requiring numerous amendments,” said the department.
“The ICT Solution will join up the clinical trial and health research whole-of-life cycle into one operational system (from approval, registration and conduct through to the publication of results) supported by jurisdictional mutually agreed processes and policies.”
The department said the NOSS solution would reduce duplication and red tape and provide access to timely and accurate information on research capability, capacity and performance for all governments, industry and communities.
“This means better strategy, better research, better treatments and better health outcomes for all Australians from research that is managed within a single national system underpinned by the highest standards of integrity,” said the RFI documents.
This ICT solution design would be underpinned by the principles of “information entered once, used multiple times” and “all data enables system-generated reporting capability”, said the department.
“The solution will have high fault tolerance and scalability that allows for the adoption of changes to existing and new workflows; information sharing; profile creation; document management (in all file formats, including video formats); internal system enabled user communication and collaboration; visibility of the approval and research management process, date stamped with dashboards and automated data and reporting for various users.”
The one-stop shop would be available to any organisation conducting health and medical research in Australia and would be mandatory for clinical trial activities required by both the Therapeutic Goods Administration and the Office of the Gene Technology Regulator.
It will replace existing state and territory research application systems, estimated to reduce duplication by:
- more than 50% in the application process;
- 35% in the Clinical Trials Notification process;
- 60% in trial registration.
Users will be able to track the progress of a clinical trial – from research application to ethics approval and site authorisation.
The NOSS project is due to start in 2026-2027 with an overall 10-year estimated delivery timeframe.
Closing date for the RFI is 2pm Canberra time on Friday 14 November 2025.
Grant opportunity: National Joint Replacement Registry
The DoHDA has also released a $13.38 million grant opportunity for the funding of a national body to be responsible for the administration of the National Joint replacement Registry. Only one organisation – the Australian Orthopaedic Association – has been invited to apply.
Running the registry involves collection, dissemination and monitoring of information relating to hip, knee, shoulder, elbow, wrist, ankle and spinal disc replacement from all hospitals in Australia that undertake joint replacement surgery.
Related
“The information is used to inform surgeons, health care professionals, governments, orthopaedic device manufacturing companies and the community on the performance of individual joint replacement devices,” said the GO documents.
“By using the best performing joint replacement devices, patients are less likely to need revision surgery.”
The department said it had reviewed the sector and determined that the AOA was the “only organisation with the demonstrated capacity and reliability to receive funding under this Grant Opportunity”.
“The AOA has considerable expertise and experience in managing and delivering quality outcomes and the current organisation responsible for maintaining the NJRR.
“The AOA founded the register in 1998 and have been maintaining it since that time. In 2009, the Commonwealth has funded the AOA, through grants, to continue with and build on the NJRR.
“During this time, the AOA have continued to produce all deliverables on time and provide a value for money service … the rate of revision surgery for knee and hip replacement has declined since the inception of the register,” said the department.
The $13.38 million is available over four years with $3.259 million available in 2025-26, $3.326 million in 2026-27, $3.391 million in 2027-28, and $3.405 million in 2028-29.